组内消息
2021-06-11
二次有机气溶胶(SOA)是大气细颗粒物(PM2.5)的重要组分,对能见度和人体健康等有重要影响,在空气污染和全球气候变化中起着关键作用。SOA主要由挥发性有机物(VOC)的降解产物通过气-粒转化生成,其化学成分非常复杂,分子组成往往可达千种。目前SOA演化机制的不确定性,直接制约了正确评估气溶胶对区域空气质量乃至全球气候的贡献影响,因而准确模拟SOA的生成和演化成为全球特别是我国所面临的重要的前沿科学问题。
中科院大气所徐永福课题组通过光化学烟雾箱实验对不同有机物氧化生成SOA的条件和化学反应机制进行了深入研究,如确定了小分子有机物生成SOA的条件和机理,确定了湿度在芳香烃和萜烯类物质生成SOA中的不同作用等。近年来,虽然国内外研究者在SOA成核、传质和老化等方面取得了很多进展,但由于缺乏一种化学动力学模式对各个过程进行耦合和评估,从而导致对SOA的认识存在碎片化的问题。
为了全面了解SOA的生成和演化规律,最近贾龙和徐永福开发了一种基于黏度的二次有机气溶胶核-壳动力学模式(CSVA)。该模式依气溶胶的分子组成将单个颗粒分为核壳两部分,并通过单一方程描述了气-粒转化涉及的气相扩散、界面传质和颗粒相扩散过程(图1),最终用化学反应动力学类似的方程对成核、传质和老化过程进行描述(图2)。烟雾箱实验表明,CSVA可以很好地捕捉以下过程:(1)依赖于湿度的H2SO4-NH3-H2O酸碱成核过程;(2)依赖于颗粒物粒径的无机和有机气溶胶吸湿动力学过程;(3)相对湿度对SOA生成的作用;(4)基于黏度的气溶胶粒径分布特征,等。此外,CSVA模式首次阐明了为什么SOA粒子会由单峰模态演变为双峰模态(图3)的内在机制。
目前国际上仅有个别基于黏度的SOA气-粒传质模型,此类模型假定SOA颗粒为多层和双层结构。这类层结构模型需要耦合各层之间的传质过程,因此极大地增加了模拟复杂度。与之相比,CSVA的结构更清晰和简洁、计算效率更高。这使得CSVA有潜力与其他空气质量模式进行耦合,从而对不同的大气条件下气溶胶演化进行准确模拟。该工作已经在Science of the Total Environment发表。
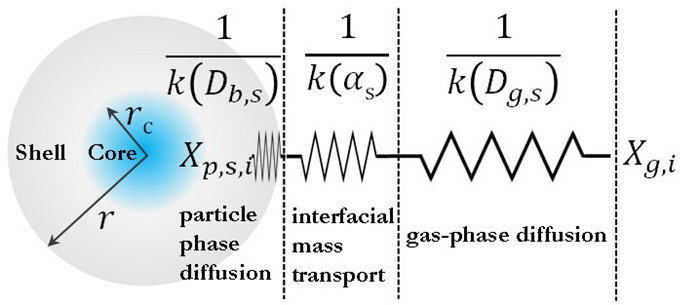
图1. 核-壳结构的单颗粒SOA的传质示意图
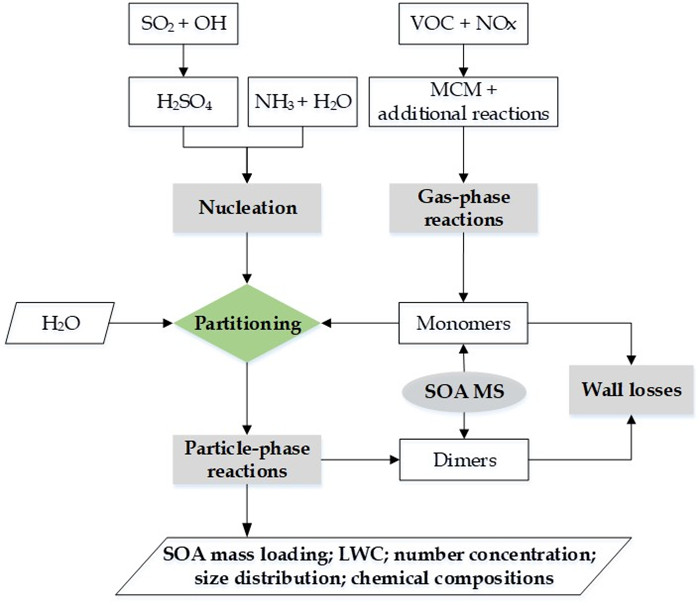
图2. CSVA模式的整体框架
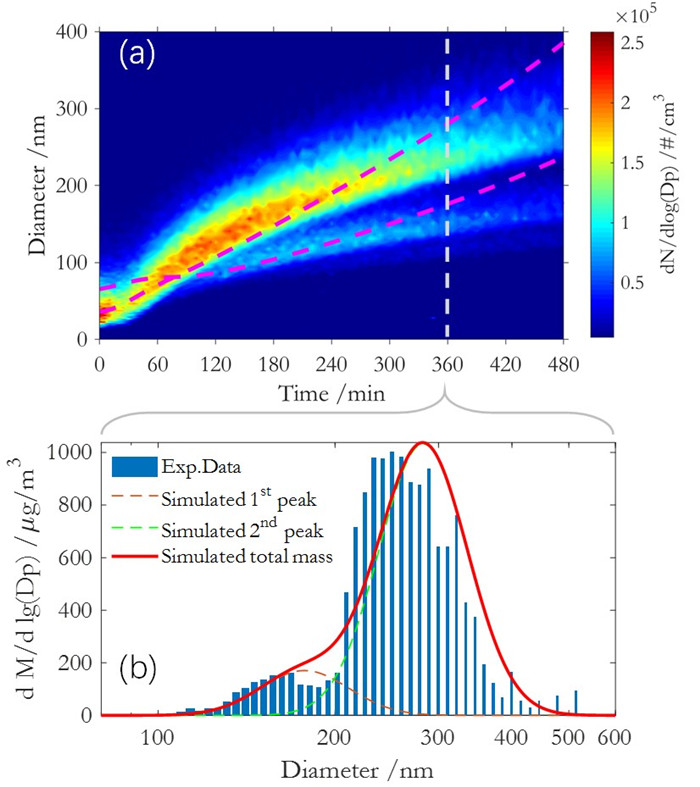
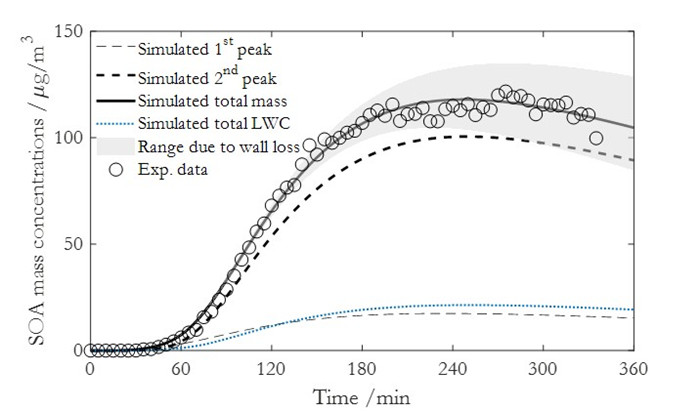
图3.CSVA模拟结果与烟雾箱实验值的比较
Jia, L., Xu, Y.F.*, 2021. A core-shell box model for simulating viscosity dependent secondary organic aerosol (CSVA) and its application. Sci. Total Environ. 789, 147954. https://doi.org/10.1016/j.scitotenv.2021.147954
Jia, L., Xu, Y.F.*, 2020. The role of functional groups in the understanding of secondary organic aerosol formation mechanism from α-pinene. Sci. Total Environ. 738, 139831. https://doi.org/10.1016/j.scitotenv.2020.139831
Zhang, Q., Xu, Y.F.*, Jia, L., 2019. Secondary organic aerosol formation from OH-initiated oxidation of m-xylene: effects of relative humidity on yield and chemical composition. Atmos. Chem. Phys. 19, 15007–15021. https://doi.org/10.5194/acp-19-15007-2019
Jia, L., Xu, Y.F.*, 2018. Different roles of water in secondary organic aerosol formation from toluene and isoprene. Atmos. Chem. Phys. 18, 8137–8154. https://doi.org/10.5194/acp-18-8137-2018
Ge, S.S., Xu, Y.F.*, Jia, L., 2017. Secondary organic aerosol formation from propylene irradiations in a chamber study. Atmos. Environ. 157, 146–155. https://doi.org/10.1016/j.atmosenv.2017.03.019
组内消息
2021-06-11
乙醛是大气环境中重要的含氧的挥发性有机物(OVOC),是臭氧生成的重要前体物。目前已有研究表明,在部分城市地区乙醛对臭氧污染的贡献已经高于其他OVOC。然而,目前关于乙醛光化学反应的烟雾箱实验研究较少,仅有的实验研究主要关注在低相对湿度(RH)条件下不同光照强度对乙醛光化学反应臭氧生成的影响,而关于RH和反应前体物浓度比值的影响规律目前仍未见报道。此外,目前广泛应用的近显式化学反应机制MCM(Master Chemical Mechanism)对乙醛光化学反应的模拟准确性仍然有待评估,并且由于乙醛同样是其他VOC物种光化学反应的中间产物,因此MCM机理对乙醛光化学反应模拟的准确性显得尤为重要。本研究结合烟雾箱实验和数值模拟方法对乙醛光化学反应过程进行了详细研究。
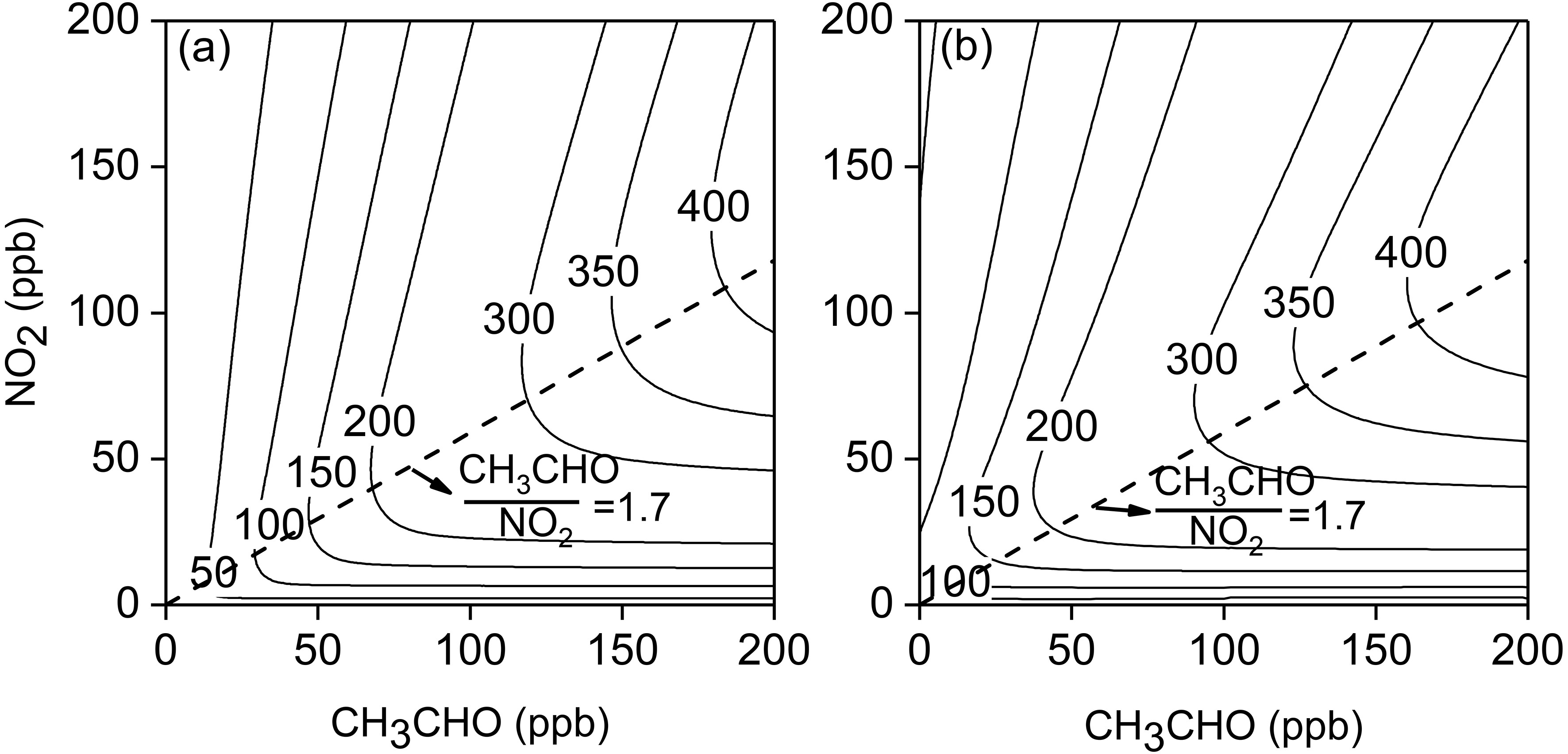
阅读剩余部分...
组内消息
2014-01-13
结合光化学烟雾箱实验与数值模拟研究了苯和乙苯在NOx存在条件下的光氧化臭氧生成潜势. 重复实验表明,在乙苯-NOx反应体系中,反应物初始浓度、温度、湿度和光照强度接近的条件下,整个反应过程中臭氧的最大偏差仅为4%,证明了烟雾箱的可重复性较高. 在烟雾箱实验的基础上,使用MCM (master chemical mechanism)模拟了苯和乙苯的光氧化O3生成,并将其结果与实验数据进行了比对分析. 干燥(≤5%)时MCM对苯和乙苯的模拟结果与实验结果较接近,如在苯-NOx反应体系中,MCM模拟的O3峰值比实验值偏大20%;在湿度为5%~70%时,MCM模拟的乙苯光氧化O3峰值与实验值偏高约(10%~25%). 用MCM模拟了太阳光照条件下苯和乙苯的臭氧生成等值线,得到在它们浓度为(10~50)×10-9,NOx在(10~100)×10-9时,苯和乙苯的6 h臭氧贡献值分别为(3.1~33)×10-9和(2.6~122)×10-9,臭氧峰值范围分别是(3.5~54)×10-9和(3.8~164)×10-9. 此外,模拟得到苯和乙苯的最大增量反应活性(maximum incremental reactivity,MIR)值分别为0.25/C和0.97/C (每单位碳). 该结果与Carter通过SAPRC机制得到的MIR值趋势一致. 模拟得到苯和乙苯的最大臭氧反应活性(maximum ozone reactivity,MOR)分别为0.73/C和1.03/C. 苯的MOR值远高于Carter使用SAPRC得到的结果,说明根据Carter得到的苯MOR会低估苯的O3潜势.
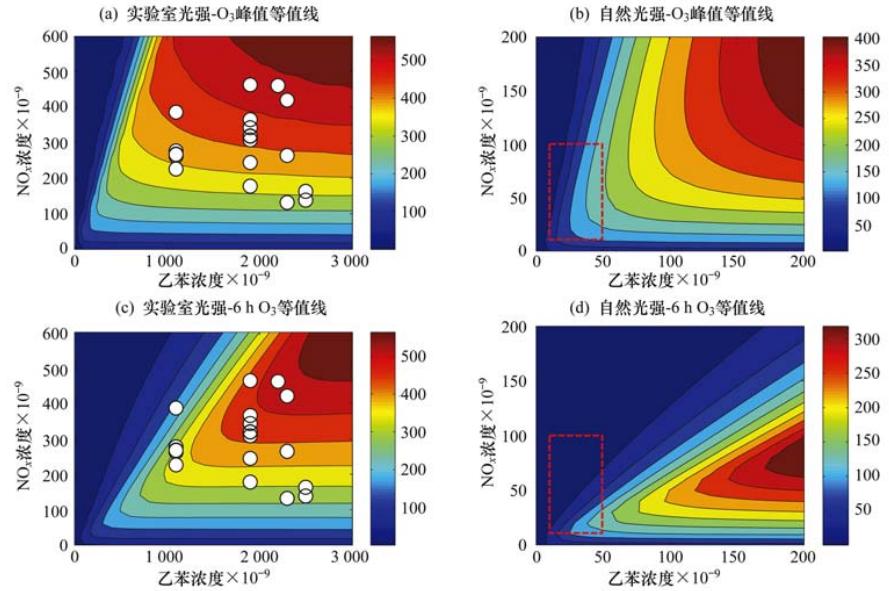
贾龙, 徐永福. 烟雾箱与数值模拟研究苯和乙苯的臭氧生成潜势. 环境科学, 2014,35(2):495~503 全文下载
Studies of ozone formation potentials for benzene and ethylbenzene using a smog chamber and model simulation
Jia Long,Xu Yong fu
Ozone formation potentials from irradiations of benzene-NOx and ethylbenzene-NOx systems under the conditions of different VOC/NOx ratios and RH were investigated using a characterized chamber and model simulation. The repeatability of the smog chamber experiment shows that for two sets of ethylbenzene-NOx irradiations with similar initial concentrations and reaction conditions, such as temperature, relative humidity and relative light intensity, the largest difference in O3 between two experiments is only 4% during the whole experimental run. On the basis of smog chamber experiments, ozone formation of photo-oxidation of benzene and ethylbenzene was simulated in terms of the master chemical mechanism (MCM). The peak ozone values for benzene and ethylbenzene simulated by MCM are higher than the chamber data, and the difference between the MCM-simulated results and chamber data increases with increasing RH. Under the conditions of sunlight irradiations, with benzene and ethylbenzene concentrations being in the range of (10-50)×10-9 and NOx concentrations in the range of (10-100)×10-9, the 6 h ozone contributions of benzene and ethylbenzene were obtained to be (3.1-33)×10-9 and (2.6-122)×10-9, whereas the peak O3 contributions of benzene and ethylbenzene were (3.5-54)×10-9 and (3.8-164)×10-9, respectively. The MCM-simulated maximum incremental reactivity (MIR) values for benzene and ethylbenzene were 0.25/C and 0.97/C (per carbon), respectively. The maximum ozone reactivity (MOR) values for these two species were obtained to be 0.73/C and 1.03/C, respectively. The MOR value of benzene from MCM is much higher than that obtained by carter from SAPRC, indicating that SAPRC may underestimate the ozone formation potential of benzene.