组内消息
2016-06-12
Effect of particle water on ozone and secondary organic aerosol formation from benzene-NO2-NaCl irradiations
(颗粒水对苯光氧化生成臭氧和二次有机气溶胶的影响规律)
Highlights
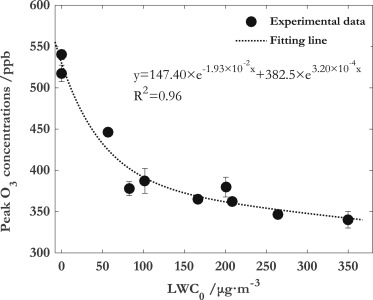
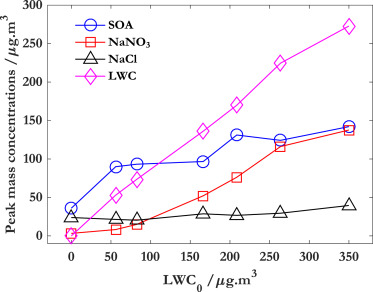
Liquid water content (LWC) can affect the ability in forming O3 and SOA from benzene.
Heterogeneous reaction of N2O5 with H2O(aq) is the major reason for the decrease of O3.
Hydrates from glyoxal were the major contributors to SOA under high LWC conditions.
Wang, Y.; Luo, H.; Jia, L.*; Ge, S. Effect of particle water on ozone and secondary organic aerosol formation from benzene-NO2-NaCl irradiations. Atmos. Environ. 2016, 140, 386–394.
Available online
组内消息
2016-02-14
High light:Ethyne is the lightest of the non-methane hydrocarbons, whose oxidation product, glyoxal, is an important precursor of secondary organic aerosol. This study explores the effects of relative humidity on the formation of secondary organic aerosol under irradiation in the presence of nitrogen oxides and sodium chloride. Results show that relative humidity can enhance aerosol formation, which provides evidence of the contribution of ethyne to organic particles.
FTIR spectra of SOA from ethyne
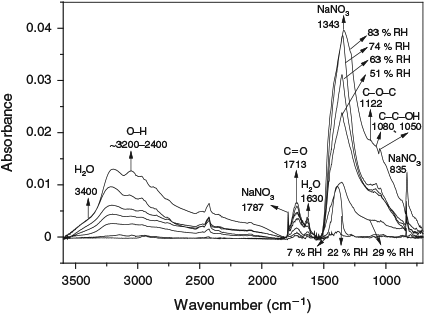
The heterogeneous photochemical oxidation of ethyne was investigated under different relative humidity (RH) conditions in the presence of nitrogen oxides and sodium chloride in a self-made indoor smog chamber. The purpose was to study the influence of RH on the formation of secondary organic aerosol (SOA) from C2H2. Through the experiments, we found that SOA was rarely formed at <22% RH in the presence of NaCl seed particles, and that SOA began to be formed at ≥29 % RH in the presence of NaCl, which shows the importance of RH in the formation of SOA. The yield of SOA (YSOA) from C2H2 was 0.2 % at 51 % RH, and increased by a factor of 17.5 as RH reached 83%. The SOA yield increased with increasing RH. The geometric mean diameter of the particles increased by a factor of 1.17, 1.22, 1.28 and 1.51 at a RH of 51, 63, 74 and 83% respectively at the end of the experiment, indicating that the growth of the particle size also increased with increasing RH. Analysis of the SOA with Fourier-transform infrared (FTIR) spectrometry indicated that the particles generated from C2H2 contained the functional groups –OH, C=O, C–O–C and C–C–OH, for whose absorption peaks increase with increasing RH.
Ge Shuangshuang, Xu Yongfu, Jia Long (2016) Secondary organic aerosol formation from ethyne in the presence of NaCl in a smog chamber. Environmental Chemistry 13, 699-710.
Full TEXT
组内消息
2015-09-11
High light:
液态海盐颗粒可以减少乙烯光氧化生成的臭氧
小分子乙烯降解产物可以在颗粒水中生成二次有机气溶胶
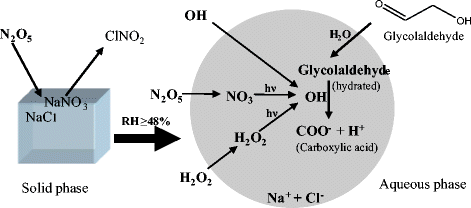
The formation of ozone and secondary organic aerosol (SOA) from ethylene-NO x -NaCl(aerosol) irradiations was studied under various relative humidity (RH) conditions in an indoor smog chamber. In the absence of NaCl seed aerosols, SOA was hardly formed and peak O3 concentrations decreased linearly with increasing RH in ethylene irradiations. For the irradiations with NaCl seed aerosols, when RH <48% (efflorescence relative humidity of NaCl), NaCl existed as solid phase and had little effect on peak O3 concentrations. The infrared spectra from sampled particles showed that SOA was rarely formed on solid NaCl particles. However, when NaCl was in the aqueous phase as RH ≥ 48%, the peak O3 concentration was sharply reduced by over 20 % as compared to experiments without NaCl aerosol, and the absorption of NaNO3 in aerosols was coincidently increased with RH. Model results indicated that the heterogeneous reaction of N2O5 with aqueous NaCl aerosols was the main cause for the sharp decrease of O3. Besides, the absorptions from C-H, C = O, ONO2 and COO groups all greatly increased with RH. Our results show that SOA from ethylene-NOx irradiations was mainly formed through aqueous reactions. The yields of SOA from ethylene were measured to be 1.5 and 2.3% at RH of 65 and 84%, respectively.
Jia, L.; Xu, Y. Ozone and secondary organic aerosol formation from Ethylene-NOx-NaCl irradiations under different relative humidity conditions. J. Atmos. Chem. 2016, 73(1):81-100; doi:10.1007/s10874-015-9317-1
full text
- « PREV
- 1
- ...
- 5
- 6
- 7
- 8
- 9
- NEXT »