组内消息
2024-09-29
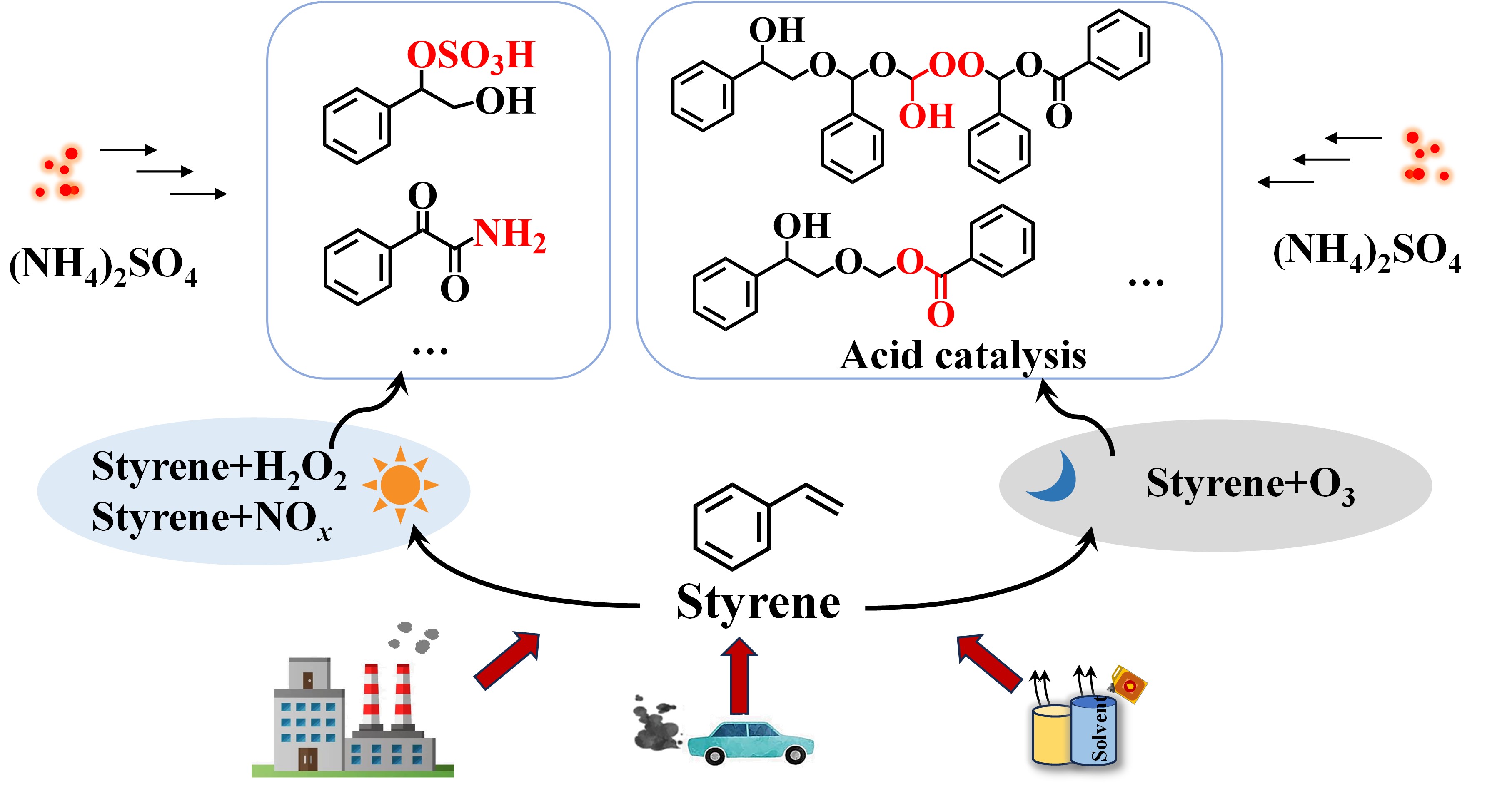
以硫酸铵等无机盐为代表的二次无机气溶胶(SIA)和二次有机气溶胶(SOA)是大气细颗粒物PM2.5的主要组成。由于SOA的成分极其复杂,因而过去的研究往往集中于SOA的生成和演化,很少关注SIA与SOA之间的相互作用。硫酸铵与SOA相互作用可能会改变气溶胶的毒性和光学特性,因此,忽略SIA与SOA之间的交叉反应,会限制我们对PM2.5理化特性的全面了解。
课题组利用实验室模拟和高分辨轨道阱质谱技术,发现烯烃降解过程中克氏中间体自由基之间存在普遍的交叉反应过程,并最新发现硫酸铵与SOA之间也存在着显著的交叉反应。研究团队分析了在不同环境条件下,硫酸铵与苯乙烯氧化产生的SOA之间交叉反应的分子组成,结果表明,铵盐主要通过颗粒相反应生成含氮有机物,而硫酸盐则主要参与形成有机硫酸酯。此外,硫酸铵的存在显著改变了苯乙烯克氏中间体生成低聚物的反应路径。这项研究不仅加深了我们对SOA形成机制的认识,突显了SIA与SOA分子间相互作用的重要性。硫酸铵与SOA相互作用会生成含氮和含硫有机物这一发现,对于揭示城市大气中PM2.5的毒性和光学特性具有重要的科学意义。
上述研究成果近期发表于期刊《Science of the Total Environment》,论文第一作者为于姗杉博士,通讯作者贾龙研究员,合作者包括徐永福研究员和潘月鹏研究员。
Yu, S.S., Jia, L.*, Xu, Y.F., Pan, Y.P., 2024. Molecular interaction between ammonium sulfate and secondary organic aerosol from styrene. Sci. Total Environ., 954,176414, https://doi.org/10.1016/j.scitotenv.2024.176414
组内消息
2024-04-08
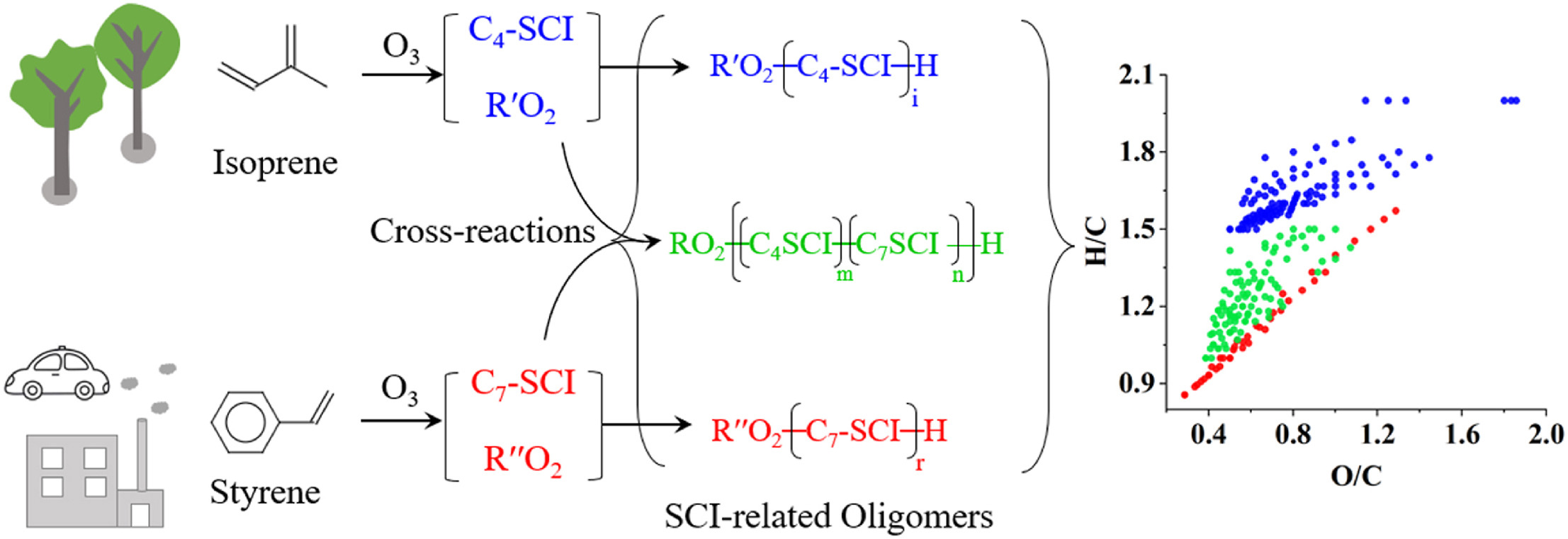
二次有机气溶胶(SOA)是大气细颗粒物的重要组成部分,会对环境、气候和人体健康产生影响。苯乙烯(styrene)和异戊二烯(isoprene)是人为和自然源SOA的重要前体物,在大气环境中广泛存在,但它们间的交叉反应并未受到关注。课题组基于烟雾箱模拟实验和高分辨轨道阱质谱技术(Orbitrap MS),研究了由苯乙烯和异戊二烯产生的克氏自由基中间体(Criegee SCIs)之间的交叉反应,以及交叉反应对SOA的形成和物理化学性质的影响规律。发现来自苯乙烯的特征C7-SCI和异戊二烯的特征C4-SCI可以发生交叉反应,并导致混合体系的SOA产率低于单一苯乙烯-O3体系,但高于单一异戊二烯-O3体系。同时在外场环境采集的细颗粒物中也发现了SCI相关的交叉产物。该研究证实SCI在决定苯乙烯-异戊二烯混合体系SOA的生成和理化性质方面的起着关键作用,说明在城市环境中,人为和自然源挥发性有机化合物(VOCs)的相互作用会显著改变SOA的理化性质,突显了交叉反应的重要性。
Yu, S.S., Jia, L.*, Xu, Y.F., Pan, Y.P., 2024. Oligomer formation from cross-reaction of Criegee intermediates in the styrene-isoprene-O3 mixed system. Chemosphere, 349, 140811, https://doi.org/10.1016/j.chemosphere.2023.140811
组内消息
2023-01-20
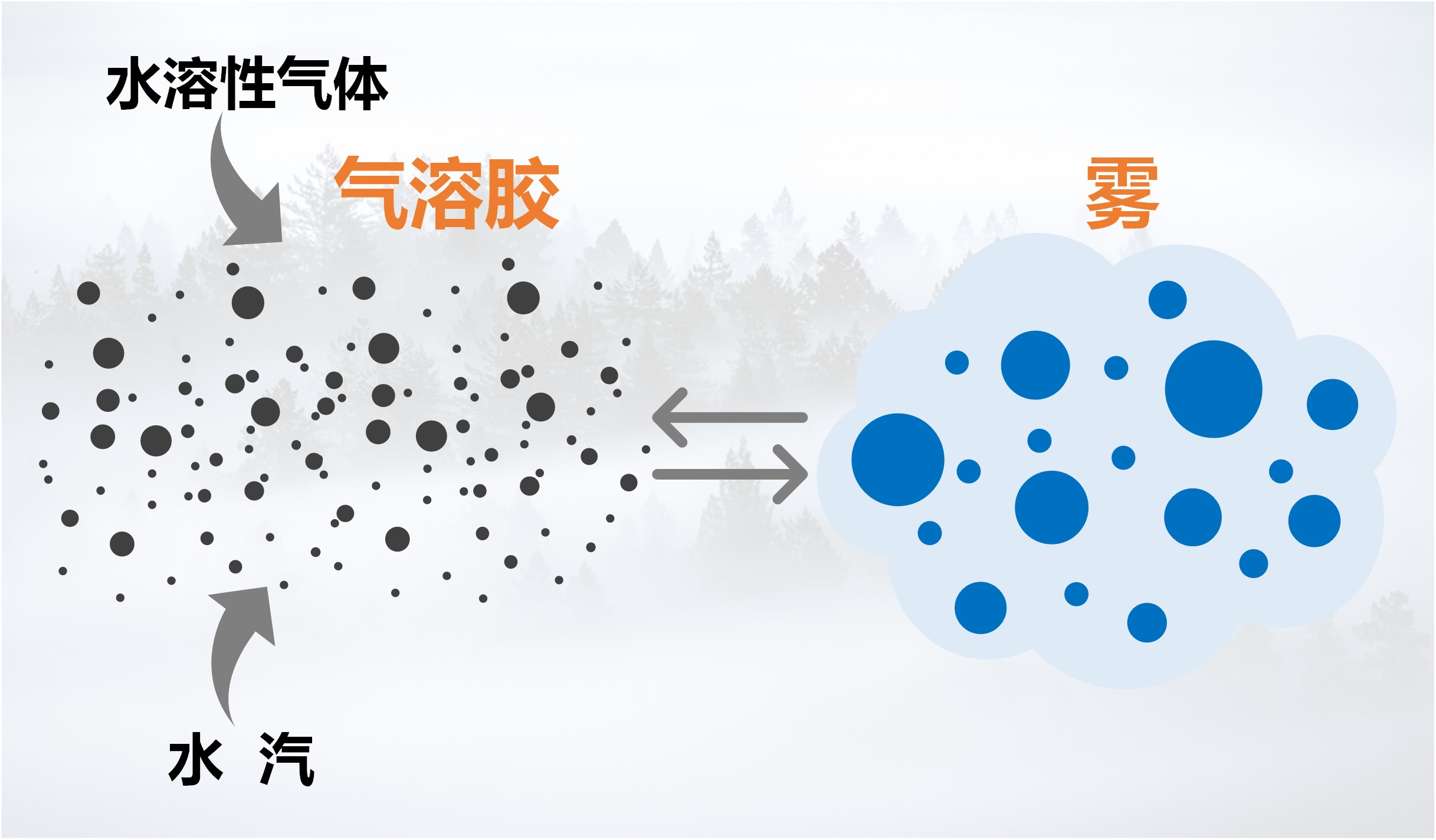
在我国秋冬季节,雾霾事件时有发生。二次有机气溶胶(SOA)对雾霾有重要贡献,然而SOA与雾(或云)之间的基本相互作用还知之甚少,主要原因包括:(1)与无机盐不同,SOA主要由半挥发或中等挥发的有机气体通过气-粒转化形成,这意味着SOA的吸湿性(或活化为云雾滴的能力)不仅取决于颗粒态有机分子,同时还受SOA气态前体物的影响;(2)SOA组成和形成机理的复杂性,SOA由成千上万种有机分子组成,其来源不仅包括各种气相反应,而且还包含了大量颗粒相反应;(3)外场观测中通常采用热力学模型(例如,ISORROPIA或E-AIM)估算颗粒水含量,但其往往忽视了有机气溶胶对颗粒水的贡献;(4)要揭示SOA-雾相互作用,还需要精细化的气溶胶动力学模式与云雾微物理过程进行耦合,而在线耦合数千反应和数百个物种的大气化学动力学过程与云雾的微物理过程是一项挑战性任务。
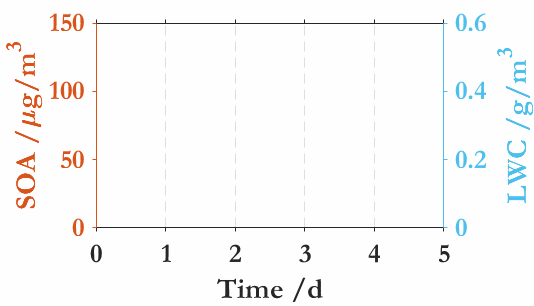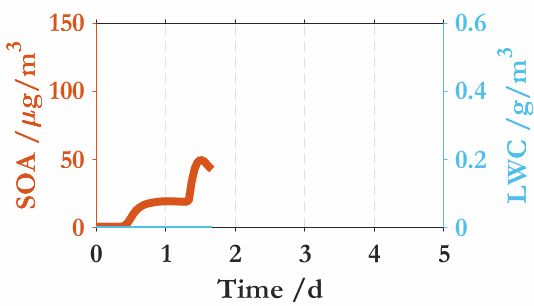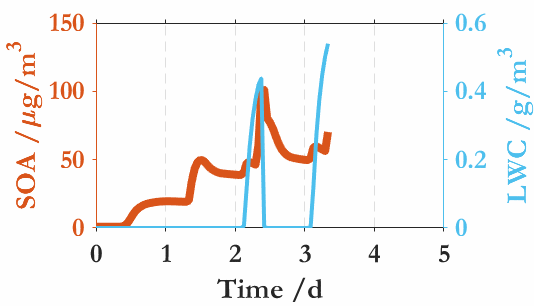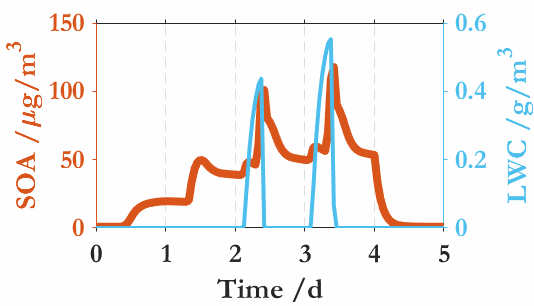
中国科学院大气物理研究所贾龙副研究员及其合作者(徐永福和段民征研究员)通过高分辨质谱技术和精细化的气溶胶动力学模式(CSVA),在深入解析甲苯气态和颗粒态分子的基础上,从云雾微物理角度解析了SOA与云雾之间的相互作用,并首次发现气溶胶-雾相互作用是导致的SOA爆发性增长的关键机制。即,霾(haze)与雾(fog)紧密交织在一起,一方面,水溶性气态有机物的存在会降低SOA活化为云雾滴的临界过饱和比,导致SOA在湿度接近或低于100%时即可活化为云雾滴;同时,云雾的生成进一步促进水溶性气态有机物的气-粒转化,从而导致了SOA的爆发式增长。还进一步分析了温度和相对湿度在SOA生成中的协同作用,发现低温可以显著放大有机气体对SOA爆发式增长和云凝结核活化的作用。上述结果表明云雾微物理过程是水溶性气态有机物快速转化为二次有机气溶胶的主要原因。
Jia, L., Xu, Y., Duan, M., 2023. Explosive formation of secondary organic aerosol due to aerosol-fog interactions. Sci. Total Environ. 866, 161338. https://doi.org/10.1016/j.scitotenv.2022.161338
- « PREV
- 1
- 2
- 3
- 4
- 5
- 6
- ...
- 19
- NEXT »